July 18, 2018

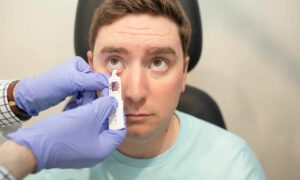
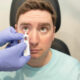
The University of Iowa Healthcare has become the first healthcare organization to implement IDx-DR, the first autonomous artificial intelligence diagnostic system cleared by the U.S. Food and Drug Administration, according to reporting from Bill Siwicki in Healthcare IT News.
The AI system, from vendor IDx, can detect diabetic retinopathy via medical imaging.
In April, IDx-DR became the first autonomous AI diagnostic system cleared by the FDA, meaning it uses artificial intelligence to make a diagnostic assessment without requiring a clinician to interpret the image or results. This enables healthcare providers who are not normally involved in eyecare to test for diabetic retinopathy, a leading cause of blindness, during routine office visits.
Caregivers at the Diabetes and Endocrinology Center at UI Health Care–Iowa River Landing in Coralville, Iowa, began using IDx-DR to assess patients for diabetic retinopathy on June 12, Siwicki writes. The clinic handles approximately 7,200 diabetes and endocrinology patient visits each year. UI Health Care plans to expand the use of the AI system across the health system.