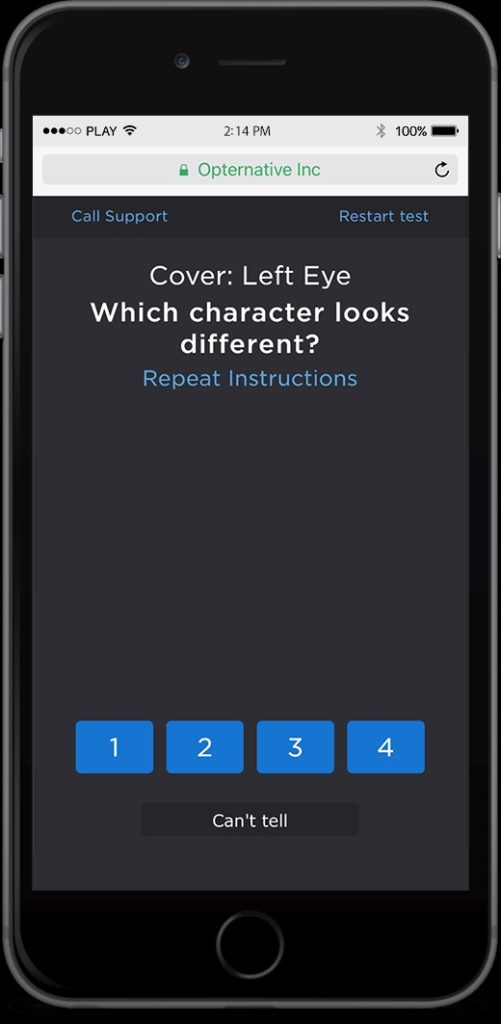
Opternative has responded to recent news reports about a warning letter from the Food and Drug Administration advising the company that its online eye examination mobile app does not have the agency’s marketing clearance or approval and is in violation of the Federal Food, Drug and Cosmetic Act, according to reporting by VMAIL.
The letter, dated Oct. 30, 2017, but released by FDA about two weeks ago, asks Opternative to submit a pre-market submission of information that would allow the agency to evaluate the app’s safety and effectiveness. It follows a similar notification from FDA that Opternative received on June 15, 2016.
Opternative issued a statement last week saying that it responded promptly to the FDA’s warning, and is “working diligently to voluntarily comply with all regulatory requirements.”
Echoing a comment that Opternative CEO Brent Rasmussen previously gave VMAIL, the company said, “We continue to communicate with the FDA on a regular basis, to work through the regulatory medical device clearance process with our outside experts.”