SPONSORED CONTENT


When silicone hydrogel (SiHy) contact lenses were introduced, they represented a major advance for patients and eye care professionals alike. SiHy lenses promote far greater oxygen transmission than conventional soft contact lens materials, an important benefit. But there’s a hitch. While silicone is advantageous for transporting oxygen through the lens, it is hydrophobic, or water-repelling, and thus attracts other hydrophobic compounds, such as tear lipids, to the lens surface.1 This can cause dry spots on the lens, leading to problems with lipid depositions (on and within the lens) and tear film instability—major causes of discomfort and poor visual performance which may lead patients to drop out of lenses.1-3 Fortunately, there is an option that minimizes these issues while still offering the breathability benefits of SiHy lenses.
The AIR OPTIX® family of SiHy soft contact lenses from Alcon feature SmartShield™ Surface Technology, the proprietary plasma surface technology that effectively inhibits the hydrophobic silicone from reaching the lens surface.4-9 This SmartShield™ Technology delivers an ultra-thin protective outer layer that helps keep moisture on the lens9 and helps keep deposits off,7,8 while helping to retain the key property of the silicone core-—breathability.10
In head-to-head tests vs. leading 2-week and monthly replacement SiHy lenses on the market, SmartShield™ has been shown to outperform the other SiHy lenses in preventing surface silicone exposure. A recent study showed that AIR OPTIX® AQUA contact lenses contained the least exposed surface silicone (<1%) vs. competitive brands.11,12
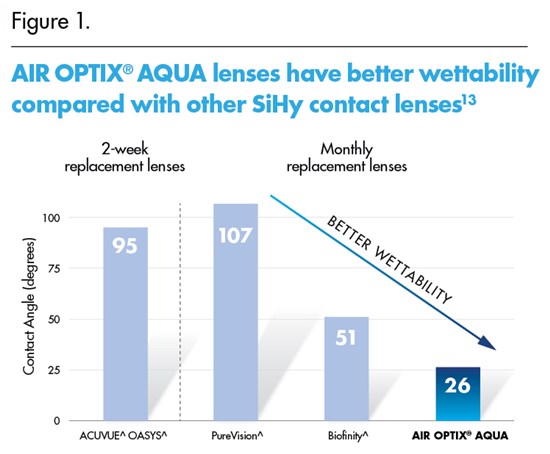
AIR OPTIX® AQUA monthly replacement lenses were also found to have better wettability than the leading comparable SiHy lenses in a “water contact angle” test on unworn lenses.13 (Figure 1)

The difference in SmartShield™ Surface Technology is clear! Now you can more quickly and easily communicate these benefits to your patients. Only AIR OPTIX® brand contact lenses have SmartShield™ Surface Technology, which delivers an ultra-thin protective outer layer that helps keep moisture on the lens9 and deposits off7,8—for clear vision and consistent comfort from Day 1 to Day 30.14 Switching my patients to AIR OPTIX® contact lenses has helped resolve complaints associated with dryness, discomfort, and diminished visual acuity, and there’s every reason to believe that AIR OPTIX® lenses with SmartShield™ Surface Technology will produce similar results for your patients.
^ Trademarks are the property of their respective owners
Important information for AIR OPTIX® AQUA (lotrafilcon B) contact lenses, AIR OPTIX® AQUA Multifocal (lotrafilcon B) contact lenses, AIR OPTIX® for Astigmatism (lotrafilcon B) contact lenses: For daily wear or extended wear up to 6 nights for near / far-sightedness. Risk of serious eye problems (i.e., corneal ulcer) is greater for extended wear. In rare cases, loss of vision may result. Side effects like discomfort, mild burning or stinging may occur.
Important information for AIR OPTIX® COLORS (lotrafilcon B) contact lenses: For daily wear only for near/far-sightedness. Contact lenses, even if worn for cosmetic reasons, are prescription medical devices that must only be worn under the prescription, direction and supervision of an eye care professional. Serious eye health problems may occur as a result of sharing contact lenses. Although rare, serious eye problems can develop while wearing contact lenses. Side effects like discomfort, mild burning or stinging may occur. To help avoid these problems, patients must follow the wear and replacement schedule and the lens care instructions provided by their eye doctor.
Important information for AIR OPTIX® NIGHT & DAY® AQUA (lotrafilcon A) contact lenses: Indicated for vision correction for daily wear (worn only while awake) or extended wear (worn while awake and asleep) for up to 30 nights. Relevant Warnings: A corneal ulcer may develop rapidly and cause eye pain, redness or blurry vision as it progresses. If left untreated, a scar, and in rare cases loss of vision, may result. The risk of serious problems is greater for extended wear vs. daily wear and smoking increases this risk. A one-year post-market study found 0.18% (18 out of 10,000) of wearers developed a severe corneal infection, with 0.04% (4 out of 10,000) of wearers experiencing a permanent reduction in vision by two or more rows of letters on an eye chart.
Relevant Precautions: Not everyone can wear for 30 nights. Approximately 80% of wearers can wear the lenses for extended wear. About two-thirds of wearers achieve the full 30 nights continuous wear. Side Effects: In clinical trials, approximately 3-5% of wearers experience at least one episode of infiltrative keratitis, a localized inflammation of the cornea which may be accompanied by mild to severe pain and may require the use of antibiotic eye drops for up to one week. Other less serious side effects were conjunctivitis, lid irritation or lens discomfort including dryness, mild burning or stinging. Contraindications: Contact lenses should not be worn if you have: eye infection or inflammation (redness and/or swelling); eye disease, injury or dryness that interferes with contact lens wear; systemic disease that may be affected by or impact lens wear; certain allergic conditions or using certain medications (ex. some eye medications). Additional Information: Lenses should be replaced every month. If removed before then, lenses should be cleaned and disinfected before wearing again. Always follow the eye care professional’s recommended lens wear, care and replacement schedule. Consult package insert for complete information, available without charge by calling (800) 241-5999 or go to myalcon.com.
References
1. Epstein AB, Stone R. Surface and polymer chemistry: the quest for comfort. Rev Cornea Contact Lens. 2010;247:15-19. Available at: http://www.reviewofcontactlenses.com/content/d/ solutions_and_lens_care/c/20136/. Accessed June 3, 2015.
2. Dumbleton K, Woods C, Jones L, Fonn D. The impact of contemporary contact lenses on contact lens discontinuation. Eye Contact Lens. 2013;39:93-99.
3. In a US survey of 1,012 contact lens dropouts aged 35-50. Alcon data on file, 2013.
4. AFM 2.5 x 2.5 micron image; Alcon data on file, 2006.
5. Alcon data on file, 2012, 2013.
6. Alcon data on file, 2013.
7. Nash W, Gabriel M, Mowrey-Mckee M. A comparison of various silicone hydrogel lenses; lipid and protein deposition as a result of daily wear. Optom Vis Sci. 2010;87:E-abstract 105110.
8. Nash W, Gabriel M. Ex vivo analysis of cholesterol deposition for commercially available silicone hydrogel contact lenses using a fluorometric enzymatic assay. Eye Contact Lens. 2014;40:277-282.
9. Alcon data on file, 2013.
10. Based on the ratio of lens oxygen transmissibilities; Alcon data on file, 2008, 2009.
11. Alcon data on file, 2013.
12. Alcon data on file, 2013
13. In vitro measurement of contact angles on unworn spherical lenses; significance demonstrated at the 0.05 level. Alcon data on file, 2009.
14. Multicenter, prospective, randomized, crossover, subject-masked, one-month clinical study of 167 subjects comparing lotrafilcon B and samfilcon A lenses. Comfort was assessed daily across the month and lenses collected for measurement of total cholesterol at the end of the month for each study period. Alcon data on file, 2015.
See product instructions for complete wear, care, and safety information.